New Discovery Uncovers the Malaria Parasite’s Achilles’ Heel
A longstanding question in malaria immunity has been resolved! In a collaborative effort led by researchers from the University of Copenhagen together with collaborators from Uganda, Tanzania, USA and Spain, the new discovery uncovers that YES, people living in regions with continuous malaria transmission develop broadly neutralizing antibodies capable of striking the malaria parasite's secret weapon and 'Achilles' heel'. This discovery could pave the way for an effective vaccine.
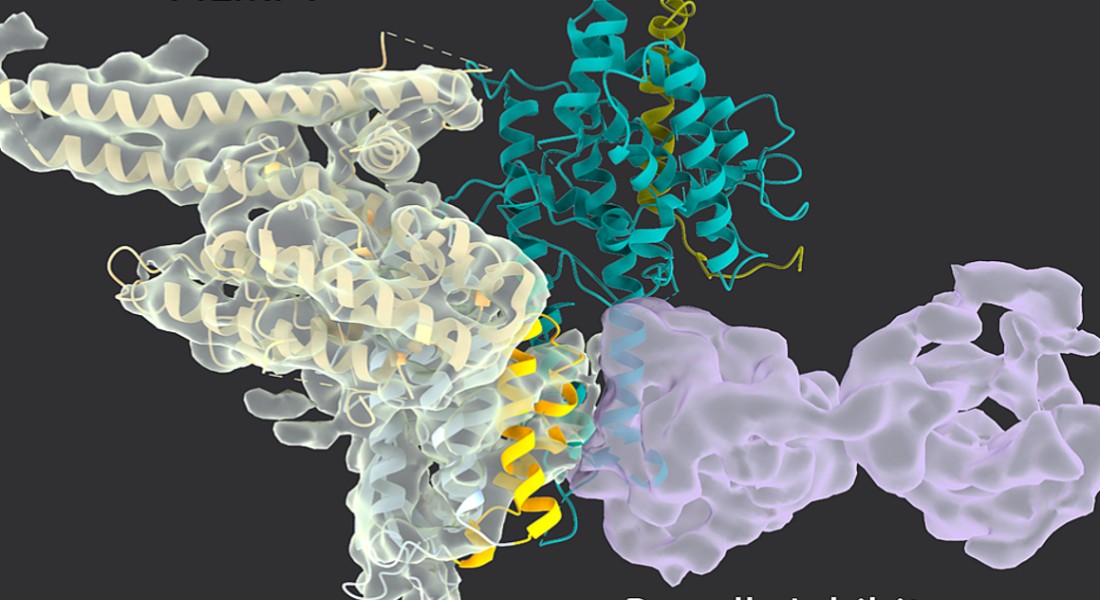
The new study, that has just been published in the prestigious scientific journal Nature, reveals the first broadly neutralizing antibodies against Plasmodium falciparum - the parasite responsible for the most severe forms of malaria and more than 600,000 death worldwide every year. Importantly, the study demonstrates how these antibodies target and neutralize a critical vulnerability in the parasite's strategy for evading the immune system.
Malaria parasites use their PfEMP1 adhesion proteins to anchor the infected red blood cells to the blood vessel walls. This prevents them from passing through the spleen, which otherwise would kill them. The parasites have many different variants of these adhesion proteins, which allows the parasite to avoid most antibody recognition. However, the newly discovered broadly neutralizing antibodies overcome this by binding to a narrowly defined region on the proteins, that the parasite cannot change without compromising its survival. This 'Achilles’ heel' provides a crucial target for neutralization and offers valuable insights that could pave the way for the development of an effective malaria vaccine
The discovery of broadly reactive antibodies
For decades, researchers have debated whether the immune system can generate broadly reactive antibodies capable of recognizing all variants of the parasite's adhesion proteins, or if achieving broad immunity is impossible due to their extensive diversity.
"After so many years of speculation and discussion, it is fantastic to finally find and prove that the broadly neutralizing antibodies do develop in immune individuals,” says Professor Thomas Lavstsen from the Centre for Translational Medicine and Parasitology (CMP) at Department of Immunology and Microbiology, University of Copenhagen, UCPH.
“We found two different antibodies from two different malaria-exposed individuals that bind the parasite’s adhesion proteins in exactly the same way – by binding a few amino acids that it cannot change. It’s a fascinating molecular insight," says Louise Turner, who is Senior scientist at both Centre for Translational Medicine and Parasitology (CMP) and Rigshospitalet.
The groundbreaking discovery is the result of an international, cross-disciplinary effort that combined expertise from multiple fields. From clinical studies of malaria-exposed populations to advanced structural and functional analyses, the collaboration brought together leading researchers from Uganda, Tanzania, Spain, the USA, and Denmark.
Towards better vaccines and treatments
The goal is to develop more effective malaria vaccines or improved treatment for severe malaria.
The breakthrough offers a promising pathway for developing improved malaria vaccines by mimicking the naturally acquired immunity seen in populations exposed to malaria. Advancements in vaccine and protein design - including novel AI technologies which were recently highlighted by the Nobel Prize in Chemistry - offer exciting opportunities.
Our idea is to mimic the immunity that naturally develops in people who live in malaria-endemic regions.
"This remains a challenge, and we need to learn more about how common these antibodies are and how they develop," adds Thomas Lavstsen and concludes:
"It is important to understand and learn from the effective strategies developed by the immune system. We cannot afford to ignore nature's own solutions."
Click here to read the article in Nature
Youtube: The Molecular interaction
In 2020 Professor Thomas Lavstsen received a Ascending Investigator Grant from the Lundbeck Foundation supporting his previous and continued studies in molecular parasitology.
Contact
Professor Thomas Lavstsen,
